Table of Contents
ToggleActivation-induced cell death (AICD) is an important regulatory mechanism of the immune system that ensures activated T lymphocytes are kept in check during and after an immune response.
What Is AICD?
When activated T cells encounter antigens, they undergo expansion, producing large numbers of effector cells to mount a powerful immune response. However, excess T cells must be eliminated once the disease threat has passed to prevent uncontrolled autoimmune reactions that could potentially begin destroying healthy tissues.
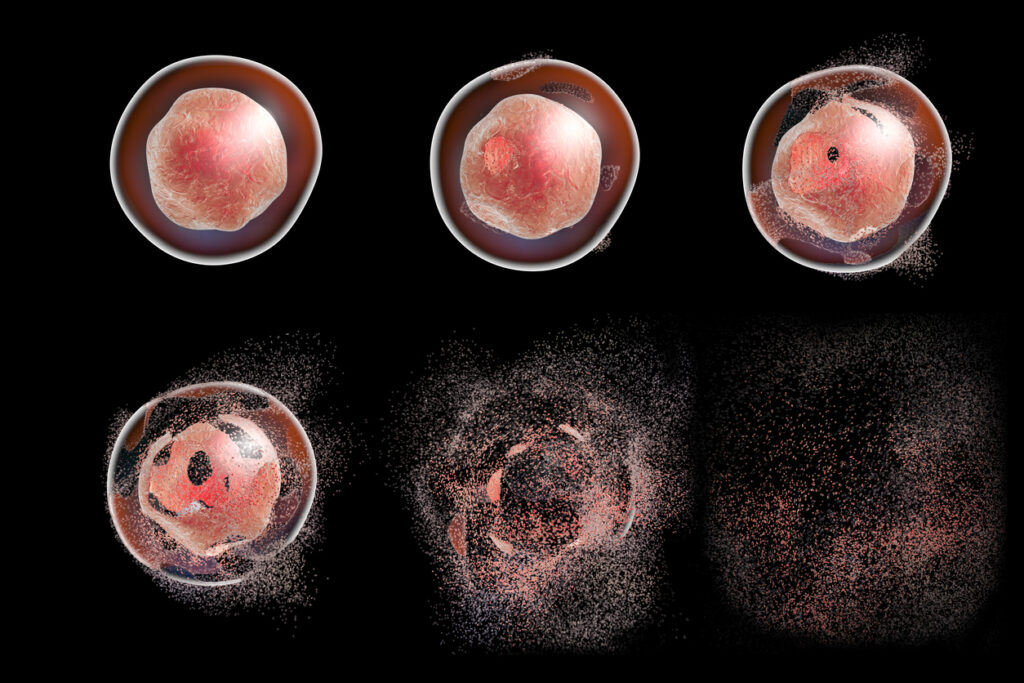
Activation-induced cell death (AICD) refers to programmed cell death, a specific type of apoptosis, of these T lymphocytes triggered by self-regulating signals. It is a crucial mechanism for maintaining immune system homeostasis and eliminating potentially harmful or self-reactive T cells. Uncontrolled self-reactive T cells can contribute to various common chronic rheumatic diseases, such as lupus, rheumatoid arthritis, and psoriasis, which often manifests as joint swelling, systemic inflammation, and scaly rashes on the body.
AICD Signaling
T cell life and death regulation is complex and involves various signaling pathways and molecules, including co-stimulatory molecules, cytokines, and the T cell receptor (TCR) complex. During an immune response, the engagement of TCR with antigen-major histocompatibility complexes (MHC) on antigen-presenting cells (APCs) initiates a series of signaling events, leading to T cell activation and subsequent effector immune functions. However, under repeated stimulation, TCR signaling can also trigger cell death by activation.
The AICD cascade for a T cell’s programmed death begins similarly to the activation of immune effector functions. The antigen-MHC activation of TCR recruits protein tyrosine kinases (such as Lck for upregulation and Fyn for downregulation) to phosphorylate immunoreceptor tyrosine-based activation motifs (ITAMs) present in the TCR-CD3 complex. This phosphorylation event initiates downstream intracellular signaling pathways. The process also involves the tumor necrosis factor (TNF) family of receptors, ceramide, and reactive oxygen species (ROS) to induce programmed cell death.
Fas/FasL Regulation
Fas (CD95) and Fas-ligand (FasL/CD95L) are membrane-bound proteins that are members of the greater TNF receptor family. Activated T cells that express FasL induce apoptosis in other cells by binding to their membrane-bound Fas as one of the major cytotoxic mechanisms of effector T cells. However, Fas/FasL interaction is also a crucial component of AICD in T lymphocytes, either through suicidal (self-induced) or fratricidal (T cell to T cell) apoptosis.
FasL binding causes Fas molecules to trimerize, recruiting adaptor molecules like FADD (Fas-associated death domain) to the newly formed Fas complex. The adaptor molecules are then bound by proteins of the caspase cascade, which ultimately induces the final stages of cell death, including DNA fragmentation, membrane blebbing, nuclear collapse, and, finally, apoptosis.
Autoregulation of Fas/FasL signaling is a delicate balance to reign in activated T cell clonal expansion while preventing excessive or inappropriate cell death that would hinder the initial immune response. Defects in FasL expression or impaired Fas-mediated signaling have been associated with autoimmune disorders and failure to eliminate self-reactive lymphocytes.
Applications of AICD in the Immune System
AICD has several important functions in the greater immune system:
- Elimination of autoreactive T cells: In the thymus, immature T cells that have not been positively selected, are self-reactive, or whose T cell receptors have not been properly rearranged will undergo apoptosis before they can join the circulating population of mature cells. If left unchecked, dysfunctional and auto-reactive T cells would likely attack the body’s own tissues, causing autoimmune disease.
- Maintenance of immune homeostasis: AICD helps eliminate excessive or unnecessary activated T cells after an immune response, preventing uncontrolled immune responses that may result in chronic inflammation and potential tissue damage.
- Prevention of lymphoproliferative disorders: AICD prevents the accumulation of abnormal or malignant T cells that cause lymphoproliferative disease and cancer, such as lymphoma or leukemia.
- Immune tolerance: AICD helps establish and maintain immune tolerance—essential for distinguishing between self and non-self antigens—by eliminating self-reactive T cells during activation.
Artificial Cell Death and CAR T-Cell Therapy for Cancer
Artificially engineered chimeric antigen receptor T cells (CAR-T cells) have become a major tool in adoptive immunotherapy for cancer. They are highly specific to the targeted cancer and do not require binding to the antigen-MHC complex to activate, as with regular T cells. However, once inside the body, CAR-T cells must navigate intrinsic AICD mechanisms to mount a successful immune response while allowing for clonal elimination to prevent unwanted side effects of overactive cells, such as cytokine release syndrome (CRS) and neurotoxicity.
Nanotein’s protein-based activation and expansion reagent technology helps to prevent premature apoptosis by strategically enriching CAR-T cells ex vivo in a less differentiated state, specifically those in the stem cell memory T cell (TSCMs) phase.
Nanotein’s NanoSpark™ STEM-T Soluble T Cell Activator allows researchers to develop CAR-T therapies that benefit from a higher percentage of TSCM cells, which have been shown to be able to rapidly differentiate into effector T cells, consistently self-renew, and resist apoptosis during the active immune response.
Contact Nanotein today to learn more.