Table of Contents
ToggleWhat Is CAR-T Cell Therapy?
CAR-T cell therapy is a groundbreaking form of cancer treatment in which a patient’s genetically modified T cells attack diseased cells, such as cancerous cells. T cells are a kind of white blood cell that plays a key role in the immune system’s response to infections and diseases.
In CAR-T therapy, T cells are collected from a patient’s blood and then engineered to express a chimeric antigen receptor (CAR) on their surface. This CAR allows the T cells to recognize and bind to specific proteins on the surface of cancer cells.
Once the CAR-T cells are infused back into the patient’s bloodstream, they can locate and destroy cancer cells that express the targeted protein. CAR-T cancer therapy has shown promising results in the treatment of certain blood cancers—such as leukemia, lymphoma, and multiple myeloma—that have not responded to other forms of treatment.
The process of creating CAR-T cells is complex and requires specialized facilities and equipment. The T cells are typically collected from the patient’s blood using a process called leukapheresis. The cells are then sent to a laboratory where they are genetically modified to express the CAR. The CAR-T cells are then expanded in number and infused back into the patient’s bloodstream.
Who Needs CAR-T Treatment?
CAR-T cell therapies are a personalized treatment approach unique to each patient’s cancer and specific needs. Given the extensive costs and clinical research currently involved with each CAR-T cancer treatment, the potential patient pool is limited. Patients who have relapsed or have refractory disease, meaning their cancer has returned after previous treatments or did not respond to previous treatments, are more likely to receive CAR-T cell therapy.
CAR-T cancer treatment has shown remarkable results in pediatric B-cell acute lymphoblastic leukemia (ALL) and non-Hodgkin’s lymphoma (NHL) that have not responded to other therapies. These types of cancer are characterized by the presence of specific protein antigens on the surface of the diseased cells that can be targeted by the CAR-T receptors.
Additionally, patients who are not candidates for other treatments like chemotherapy or stem cell transplantation due to preexisting medical conditions may also be considered for CAR-T cell therapy. However, not all patients under these umbrellas are eligible for CAR-T cell therapy. Patients must meet certain criteria, such as having adequate organ function and not having significant comorbidities that would prevent them from undergoing the treatment safely.
CAR-T Cell Therapy Process
Patient selection is not the only contributing factor to how effective CAR-T cell therapy is. The process of collecting T cells from the patient, inserting CAR encoding genes into the cells, and growing and reinfusing the cells back into the patient all play a vital role in the success of treatment.
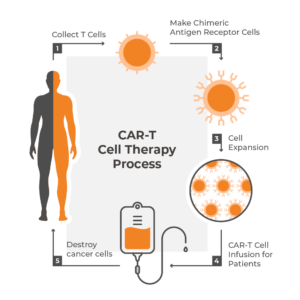
To begin treatment, white blood cells are removed in bulk from the patient in a process called leukapheresis. This procedure is done in a medical facility where two IV lines are inserted into the patient.
One line is used to remove blood from the patient and send it through an apheresis machine that separates white blood cells using centrifugal force to create a density gradient of blood cells. The appropriate density bands containing concentrated lymphocytes are removed with guidance from optical sensors and the remaining blood components are put back into the body through the other IV line.
Leukapheresis is more medically involved and expensive than the alternative of simply collecting whole blood to send to the lab. However, apheresis achieves a much higher white blood cell yield. This is particularly important in patients with low lymphocyte counts, which is typical in relapsed or refractory disease.
The process typically takes two to three hours, during which the patient will be monitored for side effects from leukapheresis, such as calcium loss. Once the procedure is complete, the white blood cells are sent to a laboratory for the CAR-T cell manufacturing stage.
Making Chimeric Antigen Receptor Cells
Upon arrival at the laboratory, T cells are isolated from the patient’s bulk white blood cell lymphocyte sample using centrifugation and elutriation or another density gradient-based cell sorting technique. Certain receptors on the T cells must then be activated to encourage the uptake of CAR-encoding materials and expand cellular numbers through replication.
Many protocols use antibodies that bind to CD3 and CD28 receptors on the T cell to induce activation. However, the application of these activators varies in format and includes other reagent components that determine which types of T cells are enriched. Nanotein’s NanoSparkTM STEM-T technology specifically targets stemlike CD8+ T cell populations to encourage expansion of T cells early in their maturation process, such as TSCM cells, which have optimal self-renewal and immune response modulation once back in the body.
There are several ways chimeric antigen receptor encoding genes may be introduced into the activated T cells. Non-pathogenic retroviral vector techniques are the most mature and well-characterized for clinical use, with proven success in permanently integrating the CAR constructs into the host cell genome.
However, there are restrictions on the size of the gene package that can be delivered with this technique. Other protocols are being studied to address this limitation using non-viral gene integration techniques, such as electroporation of transposase enzymes (Sleeping Beauty, piggyBac) or endonuclease enzymes (zinc-finger, TALENs, CRISPR/Cas9).
The newly integrated CAR-T cells are further enriched through expansion using custom combinations of growth media, serums, and cytokines. The final CAR-T therapy product is sent through a quality control process to verify sterility, identity, specificity, and potency before being cryopreserved and sent out for patient treatment.
CAR-T Cell Infusion for Patients
Once the personalized CAR-T cell therapy arrives at the host clinic, the product is infused back into the patient. The patient may go through a round of weak chemotherapy a few days before the infusion to help lower the number of native immune cells. This gives the CAR-T cells a better chance of being activated and proliferating without suppression by the native immune system.
Once CAR-T cells begin binding with cancer cells, they will rapidly proliferate and, ideally, destroy the remaining cancer cells. The patient will be closely monitored for side effects, including fever, confusion, seizures, and signs of organ damage which may be a result of cytokine release syndrome or neurotoxicity. The risk/recovery period lasts about two to three months, with special scrutiny given to the first 30 days post-infusion.
Approved CAR-T Therapies
There are six CAR-T cell therapies currently approved by the Food and Drug Administration (FDA) for the treatment of cancer. These treatments target either CD19 (a receptor found on the surface of most B cells that determines growth and development activity; often expressed by leukemia, lymphoma, and myeloma cells) or BCMA (an important signaling receptor found on mature B cells; often expressed by lymphoma and myeloma cells).
- Axicabtagene ciloleucel (Yescarta®): A CD19-targeting CAR-T cell immunotherapy that’s approved for subsets of lymphoma patients.
- Brexucabtagene autoleucel (Tecartus™): A CD19-targeting CAR-T cell immunotherapy that’s approved for subsets of leukemia and lymphoma patients.
- Ciltacabtagene autoleucel (Carvykti™): A BCMA-targeting CAR-T cell immunotherapy that’s approved for subsets of advanced multiple myeloma patients.
- Idecabtagene vicleucel (Abecma™): A BCMA-targeting CAR-T cell immunotherapy that’s approved for subsets of advanced multiple myeloma patients.
- Lisocabtagene maraleucel (Breyanzi®): A CD19-targeting CAR-T cell immunotherapy that’s approved for subsets of lymphoma patients.
- Tisagenlecleucel (Kyrmriah®): A CD19-targeting CAR-T cell immunotherapy that’s approved for subsets of leukemia and lymphoma patients.
Nanotein Offers CAR-T Cell Expansion for CAR-T Therapy
Overall, CAR-T cell therapy is a promising innovative treatment option for patients with relapsed or refractory blood cancers and limited treatment options. Ongoing research is exploring the potential of CAR-T cell therapy for other types of cancer, such as solid tumors. However, there are additional challenges to overcome, including managing the potential side effects of treatment like cytokine release syndrome (CRS) and neurological autoimmune effects.
Nanotein Technologies’ products address this from the beginning by providing the best possible CAR-T cell activation and enrichment strategy for your CAR-T therapy products. Using our NanoSpark™ STEM-T Soluble T Cell Activator, your CAR-T products will benefit from TSCM CAR-T expansion, generating large quantities of clinically potent T cells in a less differentiated state for increased self-renewal ex vivo and clinical safety during treatment.
Contact us today to learn more.

