TSCM Cells: Better CAR-T
Home » TSCM for Better CAR-T Cell Therapy
The cell composition of the final product matters when developing or manufacturing a T cell based cancer therapy such as CAR-T or TCR-T. Cell composition ultimately impacts the effectiveness and longevity following treatment, as well as the safety of the therapy itself.
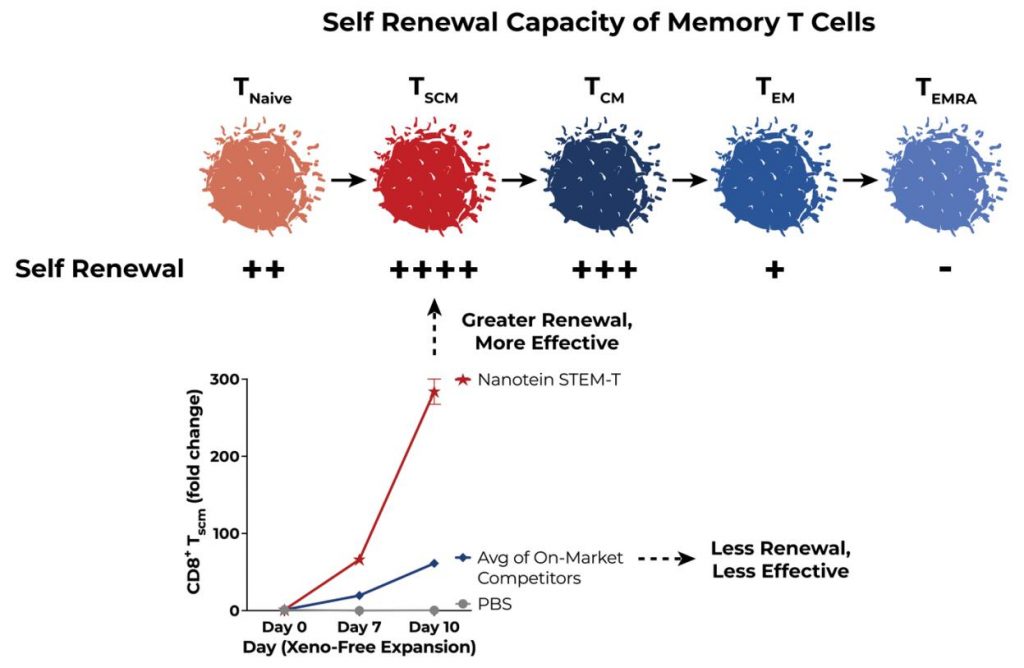
T cell based treatments that are enriched in more stemlike T cells have been shown to last longer in vivo and be more effective and safer for patients. Stem cell memory T cells, or TSCMs, are one of the most important types of these stemlike T cells. As such, targeted, intentional expansion of TSCMs and enrichment in the final adoptive T cell based product is one of the most promising strategies for making improved and better T cell based cancer therapies.
Understanding T Cell Maturity Basics
Like many types of T cells, memory T cells exist along a continuum of maturity. The developmental path of a T cell is dictated by a complex signaling network of growth factors and cytokine pathways that result in a naive T cell expressing or suppressing specific phenotypic traits during a process called differentiation. This differentiation creates functionally different subsets of T cells that perform clinically distinct roles in a patient’s immune response. The continuum of maturity for memory T cells ranges from the least differentiated functional phenotypes—naive and TSCMs —to the most inflexible phenotypes—effector memory (TEM) and terminally differentiated effector memory (TEMRA) T cells. In the last two decades, significant scientific research has gone into understanding the effect maturity state has on clinical outcomes. Less mature memory T cell states, specifically TSCM, have already shown great potential in cancer and HIV treatment.