An Overview of CAR-T Cell Expansion
Home » An Overview of CAR-T Cell Expansion
CAR-T Cell Culture for CAR-T Therapy
During CAR-T adoptive immunotherapy, a patient’s T cells are altered genetically to express chimeric antigen receptors (CARs) on their surface. CAR-T receptors bind directly to specific antigens on the surface of cancer cells or other diseased cells.
CAR-T cell expansion is the process of increasing the number of these altered cells that contain CARs. Activating and expanding these CAR-T cells is critical for the success of CAR-T therapy.
Why Is CAR-T Expansion Necessary?
CAR-T cell expansion is necessary to generate a sufficient number of modified immune cells for the treatment of disease. The required cells/kg body weight dosing varies widely based on the patient and disease characteristics, but it is not unusual for patients to require several million CAR-T cells for successful treatment.
Tisagenlecleucel is one such approved CAR-T-based blood cancer treatment with a dosing range of 0.2 to 250 million CAR-T cells/kg body weight. Recent research has shown that higher initial dosing correlates with more successful outcomes.
Types of CAR-T Expansion
CAR-T cell expansion happens inside (in vivo) and outside (ex vivo) the body in a laboratory during a typical adoptive cell therapy cycle.
Once CAR-T cells are infused back into the body, they quickly locate and concentrate around their target tumor cells, often killing them via contact-dependent cytotoxicity. This immune response induces proliferation, further expanding the number of CAR-T cells and dramatically increasing the local cytokine activity.
Long-term persistence and robust in vivo expansion of CAR-T cells are associated with sustained clinical remission and patient survival rates. However, patients undergoing this process must be closely monitored for excessive production of inflammatory cytokines that may lead to dangerous side effects, such as cytokine release syndrome (CRS). Some ways to mitigate CRS are through the engineered design of CARs, responsible reagent use, and careful selection of T-cell isolates during initial expansion ex vivo in the laboratory. Additionally, existing anti-inflammatory CRS treatments such as Tocilizumab, a monoclonal antibody (mAb) therapy that blocks IL-6 receptors, and corticosteroid are being studied as prophylatic options to prevent CRS during CAR-T therapy.
Once T cells are isolated from the patient’s body, they must be activated to allow the uptake of CAR-encoding genetic material. This can be via transduction using lentiviral vectors, or through non-viral transfection methods. Because this process happens outside of the body, next-generation T-cell manufacturing protocols are being studied to optimize activation and expansion of the T-cell culture and isolate advantageous T-cell populations, such as stem cell memory T cells (TSCM).
CAR-TSCM cells have superior in vivo antitumor activity and expansion potential and can also reduce the overall number of released cytokines compared to bulk CAR-T cell populations. Preselecting CAR-TSCM during activation and expansion will increase the CAR-T therapy quality, possibly allowing for a lower initial dose needed to achieve remission.
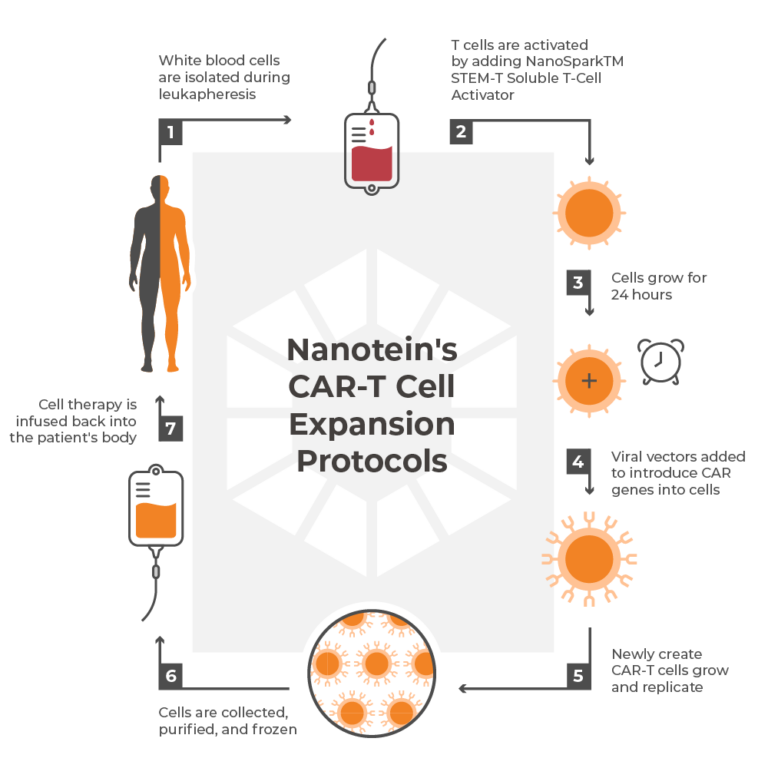
Nanotein's CAR-T Cell Expansion Protocols
Before a patient’s initial T-cell isolates are expanded ex vivo, they must first be engineered to express the desired CARs. T cells are activated in vitro using Nanotein’s NanoSparkTM STEM-T Soluble T Cell Activator, which uses anti-CD3/CD28 pathways to induce CAR gene delivery uptake in stemlike CD8+ cells. Once the genes encoding the CARs have successfully integrated into the host’s T cells, the number of cells is strategically expanded using a custom combination of antigens and cytokines that are supported by scientific literature.
Interleukin 2 (IL-2) is a potent general T-cell growth factor essential for the long-term proliferation of activated T cells. IL-2 has also been used as a standalone systemic clinical treatment for some metastatic cancers. However, IL-2 monotherapy can cause severe side effects in high doses, and efforts to further improve treatment focus on its combination with adoptive cell immunotherapies.
Interleukin 7 (IL-7) is a cytokine required in early T-cell development that promotes T-cell survival by up-regulating the expression of anti-apoptotic genes.
Interleukin 15 (IL-15) is an inflammatory cytokine that supports the homeostasis of naive CD8+ T cells. Combining IL-7 and IL-15 in culture promotes the enrichment of self-renewing T cells like TSCM.
Nanotein Is Addressing Challenges in CAR-T Cell Expansion
Many current methods of activation and expansion are inefficient and non-specific to stemlike T cells. They introduce clinical risks to the process by using synthetic ingredients and magnetic beads that may contaminate the therapy if not painstakingly removed from the expanded culture.
To address this, Nanotein Technologies developed novel protein-based CAR-T technology in a soluble format that requires less time, fewer T cells, and no magnetic components or synthetic contaminants. Nanotein’s NanoSpark™ STEM-T Soluble T-Cell Activator focuses on TSCMs CAR-T expansion, generating large quantities of clinically potent T cells in a less differentiated state for increased self-renewal ex vivo and clinical safety during treatment.
Contact us today to learn more.
