Add NanoSparkTM STEM-T Soluble T Cell Activator to your cart now!
Part Number: STEM-T
If you are purchasing outside of the United States, Canada, China, Germany, Japan, Sweden, Switzerland, Taiwan, or United Kingdom – Please contact info@nanoteintech.com to complete your purchase.
The NanoSparkTM STEM-T Soluble T Cell Activator is a culture-ready reagent for in vitro activation and expansion of human T cells. The nanoscale of the NanoSparkTM STEM-T protein complex allows for sterile filtration and removal of excess reagent via either media exchange or simple centrifugal cell washing.
NanoSparkTM STEM-T is best in class, particularly for preserving stemlike CD8+ T cells important for higher efficacy. Competing products introduce clinical risks to the process by using synthetic ingredients that may contaminate T cell therapy if not painstakingly removed from the expanded culture.
The NanoSparkTM product line is modular and plug-and-play by design, using a proprietary self-assembly protein nanoparticle complex that is inherently soluble and can be filter sterilized.
The NanoSparkTM STEM-T Soluble T Cell Activator contains self-assembling protein nanoparticles with functional antibodies bound to the surface through high-affinity non-covalent interactions. These anti-CD3 and anti-CD28 antibodies are arranged in a proprietary biophysical combination on the NanoSparkTM platform to drive best-in-class performance that favors stemlike CD8+ T cell production.
The NanoSparkTM STEM-T Soluble T Cell Activator activates resting human CD3+ T cells originating from purified T lymphocytes or patient peripheral blood mononuclear cells (PBMCs).
Need help getting started? Want to benefit most from your T cell activation and expansion? Contact the Nanotein team today to learn more.
Soluble, magnetic bead-free, protein-based, and ready-to-use.
Excellent total T cell expansion with industry-leading clinically desirable CD8+ stem cell memory T cell expansion.
Employs anti-CD3/CD28 antibodies and maintains high cell viability.
| Product Line | NanoSparkTM |
| Cell Type | T cells |
| Sample Type | PBMC, cell culture, T cell isolate |
| Reactivity | Human |
| Application | T cell activators, T cell manufacturing, immunotherapy |
| Grade | Research grade |
| Quantity | 2 mL |
| Contents | Activator contents suspended in phosphate-buffered saline glycerol |
| Functional Testing | ≥80% expected proliferation of T cells on day 7 |
| Sterility | Sterile 0.2 µm filtered post-aseptic production |
| Mycoplasma | Negative |
| Storage | Keep frozen at -80°C for long-term storage Once thawed, store at 4°C protected from light for up to one month |
| Shipping Condition | Frozen on dry ice |
| Product Weight | 13.15 g (0.0289 lbs) |
| Product Dimensions | 5 mL clear glass vial (20 mm x 22 mm x 40 mm – vial opening x vial diameter x vial height) |
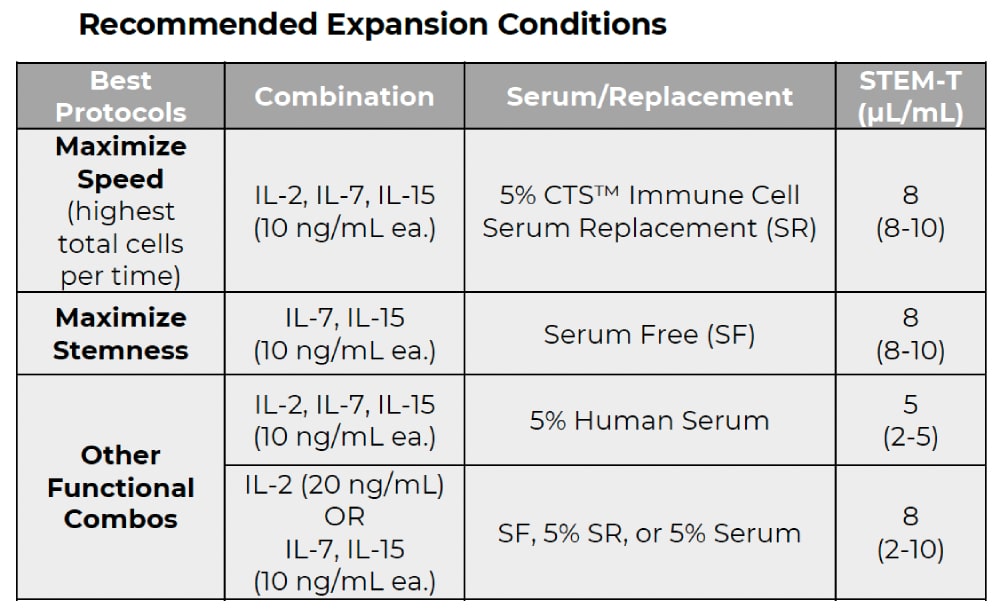
Consult the data sheets and supporting documentation for usage instructions and additional details, or explore the recommended protocols, which include suggested catalog numbers for cytokine supplements and media.
These protocols are recommended and include suggested catalog numbers of cytokine supplements and media to use.
The NanoSparkTM STEM-T Soluble T Cell Activator is used in vitro to induce activation and CAR gene delivery uptake in stemlike CD8+ cells via anti-CD3/CD28 pathways. It is intended for ex vivo activation and expansion of CD3+ T Lymphocytes (human resting T cells) from T cell isolates or peripheral blood mononuclear cells (PBMCs). The activator should be stored at -80 °C long-term. Once thawed, store at 4 °C protected from light for up to one month.
The NanoSparkTM STEM-T Soluble T Cell Activator is designed for use with a cytokine-supplemented T cell expansion medium. Suggested protocols have been developed for common cytokine-supplemented strategies. Optimization of the provided general protocol information may be necessary depending on your experimental objectives.
Standard STEM-T Expansion Protocol & Data Sheet
Short 3-Day STEM-T Expansion Protocol & Data Sheet
Lot-specific STEM-T CoA will be shipped with the product. Contact customer service if additional copies are required.
Carefully read product SDS before use.
If using transduction (optional), wait 24-72 hours after adding NanoSparkTM STEM-T Soluble T Cell Activator to the cell culture before adding the viral vector. If using electroporation, then electroporate 72 hours after activator addition.

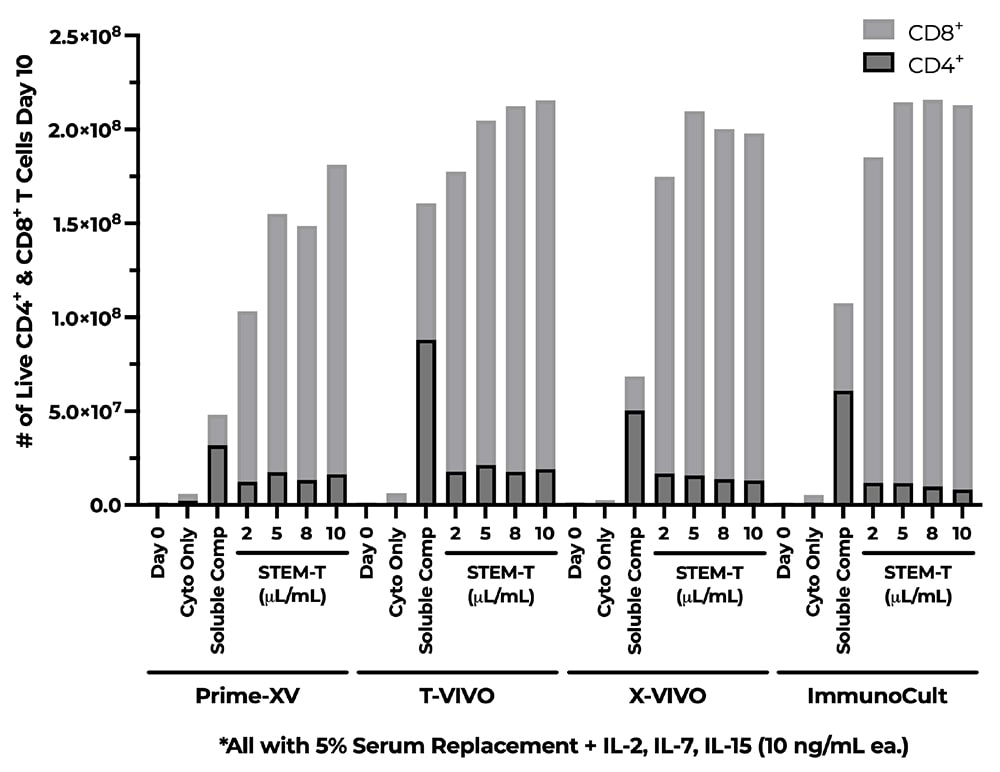
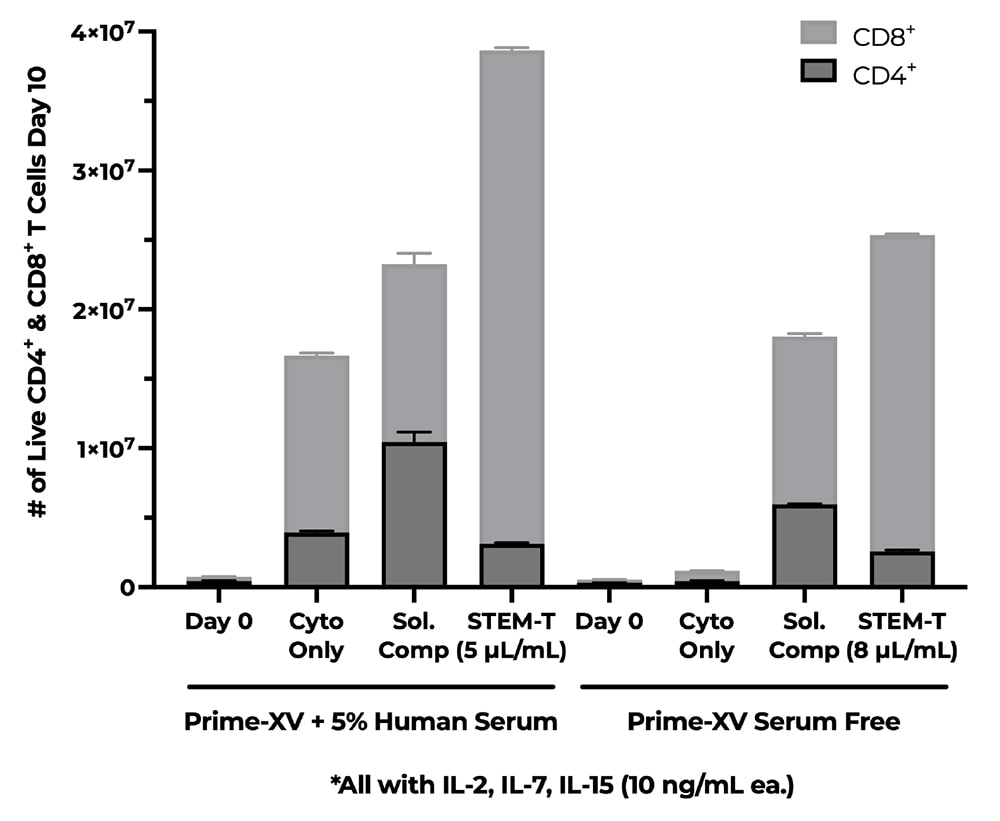
A) Fold Change of CD3+ Cells, B) % of CD4+ & CD8+ Cells, C) CD4+ & CD8+ T-Cell Phenotype Percentage. NanoSparkTM STEM-T Soluble T-Cell Activator was cultured in StemCell’s ImmunoCult-XF T Cell Expansion Medium (xeno-free) supplemented with IL-7 and IL-15. Cells were expanded for 10 days and analyzed on a flow cytometer on days 0, 7, and 10. Cells were labeled with CD4+, CD8+, CD45RA, CCR7, and CD95 fluorescent antibodies (Schmueck-Henneresse et al 2017). Expansion with NanoSparkTM STEM-T Soluble T-Cell Activator enhances total cell viability and stimulates expansion of CD8+ T-Cells and promotes the stem-like phenotypes of CD4+ and CD8+ T-Cells: Tnaive, Tscm, and Tcm.

A) Fold Change of CD3+ Cells, B) % of CD4+ & CD8+ Cells, C) CD4+ & CD8+ T-Cell Phenotype Percentage. NanoSparkTM STEM-T Soluble T-Cell Activator was cultured in StemCell’s ImmunoCult-XF T Cell Expansion Medium (xeno-free) supplemented with IL-7 and IL-15. Cells were expanded for 10 days in a G-rex6M and analyzed on a flow cytometer on days 0, and 10. Cells were labeled with CD4+, CD8+, CD45RA, CCR7, and CD95 fluorescent antibodies (Schmueck-Henneresse et al 2017). Expansion with NanoSparkTM STEM-T Soluble T-Cell Activator enhances total cell viability and stimulates expansion of CD8+ T-Cells and promotes the stem-like phenotypes of CD4+ and CD8+ T-Cells: Tnaive, Tscm, and Tcm.
Nanotein assessed the NanoSparkTM platform with adherence to rigorous ISO 10993 standards in preparation for GMP manufacturing. The composition of the NanoSparkTM reagent is protein-based, and consequently naturally biodegradable, which provides a significant safety advantage over magnetic bead-based and synthetic products. A reputable medical device CRO was commissioned to complete the in vitro & in vivo ISO 10993 biocompatibility testing. The tests were run at >20X the predicted possible carry-over amount, which resulted in no toxicity or adverse effects:
| ISO 10993 Biocompatibility Testing | Test | Type of Test | Result | Product Tested |
| ISO 10993-5 | Cytotoxicity | In vitro | No Toxicity/Adverse Effect | NanoSpark STEM-T |
| ISO 10993-10 | Sensitization | In vivo: Guinea Pig | No Toxicity/Adverse Effect | NanoSpark STEM-T |
| ISO 10993-10 | Irritation/Intracutaneous Reactivity | In vivo: Rabbits | No Toxicity/Adverse Effect | NanoSpark STEM-T |
| ISO 10993-11 | Acute Systemic Toxicity | In vivo: Mice | No Toxicity/Adverse Effect | NanoSpark STEM-T |
| ISO 10993-11 | Pyrogenicity | In vivo: Rabbits | No Toxicity/Adverse Effect | NanoSpark STEM-T |
| ISO 10993-4 | Hemocompatibility | In vitro | No Toxicity/Adverse Effect | NanoSpark STEM-T |
| ISO 10993-3 | Genotoxicity Ames | In vitro | No Toxicity/Adverse Effect | NanoSpark STEM-T |
| ISO 10993-3 | Genotoxicity Mouse Lymphoma Forward Mutation | In vitro | No Toxicity/Adverse Effect | NanoSpark STEM-T |
CD8+ T cells, also known as cytotoxic T cells or “killer T cells”, play a crucial role in the human...

CD4+ T cells, sometimes called “helper” T cells, play a more central role in the immune system than their name...

What Is Adoptive Cellular Immunotherapy? Adoptive cellular immunotherapy is a medical procedure that uses the patient’s immune cells to target...